СОР № 1 Английский язык 11ЕМН класс Aromatic compounds
НазадAromatic compounds
Задание:
1.
Describe the structure and bonding in a benzene molecule.
2.
Benzene is prepared from ethyne by the process of cyclic polymerization.
(a) Write a chemical equation for this process.
(b) Methylbenzene is the first homologue of benzene.
(i) Write an equation of obtaining methylbenzene from benzene by Friedel‐Crafts alkylation.
(ii) Which catalyst is used in this reaction?
(c) Benzene readily undergoes addition reaction in the presence of ultraviolet light (but without a catalyst present), hot benzene will also undergo an addition reaction with chlorine or bromine.
Write an addition reaction of the reaction of benzene with chlorine.
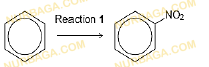
(d) Nitrobenzene can be prepared from benzene as shown.
3.
Concentrated sulfuric acid catalyses this reaction.
(a) Write an equation to show how H2SO4 generates the electrophile needed in this reaction.
(b) Draw the mechanism of the reaction
between the electrophile and benzene to form nitrobenzene. Include all relevant curly arrows and charges.
(c) Toluene and benzene are in the same homologues series. Toluene reacts about 25 times faster in nitration reaction than benzene under identical conditions.
Explain why toluene is more reactive than benzene.